Abstract
Introduction: Hypomethylating agent in combination with venetoclax (HMA+Ven) was approved for treatment of newly diagnosed acute myeloid leukemia (AML) after a phase III trial showed 66.4% CR/CRi rates and 17.8 mos median duration of response. However, these results were seen in patients 75 years and older or those who were considered unfit for intensive chemotherapy (IC). These CR rates are superior compared to what was historically reported with IC. Post-remission treatment for most fit patients with intermediate and adverse risk AML involves allogeneic stem cell transplantation (alloSCT) which offers the highest chance of maintaining long-term remission. Many fit patients still receive IC for the purpose of inducing remission. Our aim was to study the post-transplant outcomes of patients who were treated with HMA+Ven compared to CPX-351 along with the factors that help choose between IC and HMA+Ven for fit patients that are transplant candidates.
Methods: A retrospective review of patients with newly diagnosed AML treated with CPX-351 or HMA+Ven at Moffitt Cancer Center was performed. Patients who were treated with either CPX-351 or HMA+Ven as front line therapy and then proceeded to alloSCT were included in this analysis. Chi square was used to compare baseline characteristics. Survival estimates were calculated using the Kaplan-Meier method from date of transplant to the date of death or date of last follow up.
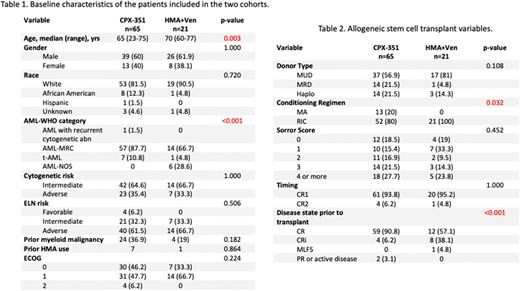
Results: Out of the total 147 patients that received CPX-351, 65 (44.2%) underwent alloSCT. In contrast, from the 216 patients treated with upfront HMA+Ven, only 21 (9.7%) patients proceeded to alloSCT (p<0.00001). The median age at diagnosis for the CPX-351 treated patients was significantly different from the HMA+Ven patients (65 vs 70 years; p=0.003). There was no difference in gender or race between the two groups. Table 1 describes the baseline and disease characteristics of the two cohorts. More patients with AML-MRC were treated with CPX-351 than HMA+Ven (87.7 vs 66.7%; p<0.001). There were no differences in the distribution of patients in the two groups based on cytogenetic risk, ELN risk or ECOG performance status.
Table 2 describes the transplant variables. There were no differences in the type of donor or timing of transplant between the two groups. No patients in the HMA+Ven group underwent myeloablative conditioning (MAC), while 20% of patients in the CPX-351 cohort underwent MAC prior to transplant (p=0.032). Looking at disease state prior to transplant, we saw that more patients in the HMA+Ven group were in CRi (38.1 vs 6.2%; p= <0.001). There were no significant differences based on the Sorror score, hence the co-morbidities between the two cohorts were not significantly different. A higher number of patients with TP53 mutations were treated with HMA+Ven prior to transplant (27.8 vs 7.9%; p=0.047) and greater number of patients with DNMT3A mutations were treated with CPX-351 (30.2% vs 0; p=0.005). There was no significant difference between post-transplant relapse rates between the CPX-351 and HMA+Ven cohorts (12/65, 18.5% vs 5/21, 23.8%; p=0.592).
At a median follow up of 18 months, the median OS for both groups was not reached. The 12-month OS probability was 71.4% for patients treated with upfront CPX-351 compared to 69.6% for upfront HMA+Ven (p=0.977). At a median follow up of 16 months, the relapse free survival was also not reached for both groups. The 12-month post-transplant RFS was 80.8% for CPX-351 vs 74.3% for HMA+Ven (p=0.548; Figure 1). The 1-year NRM was not significantly different between the two groups either (13/52, 25%, 2/19, 10.5% respectively; p=0.271)
Conclusion: In this analysis a greater number of patients proceeded to alloSCT after achieving remission with CPX-351 compared to HMA+Ven for newly diagnosed AML. Patients who were treated with HMA+Ven tended to be older than those who received CPX-351. However, there were no differences in the ELN risk, OS, RFS or rate of relapse between the two cohorts. This study is limited by the small number of patients however provides an important conclusion, noting no difference in post-transplant OS and RFS for patients who are fit to proceed to a transplant if treated with HMA+Ven or CPX-351. A prospective study with larger numbers of patients is needed to confirm these findings.
Disclosures
Kuykendall:Pharmaessentia: Consultancy, Honoraria, Speakers Bureau; Imago Biosciences: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria, Speakers Bureau; Blueprint: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; GSK - Sierra Oncology: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Prelude Pharmaceuticals: Other: Research Support; BMS: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Morphosys: Other: Research Support; Protagonist: Other: Research Support; CTI Biopharma: Consultancy, Honoraria, Speakers Bureau. Komrokji:Geron: Honoraria, Membership on an entity's Board of Directors or advisory committees; Taiho: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servio: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CTI biopharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PharmaEssentia: Honoraria, Other, Speakers Bureau; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Padron:Kura: Research Funding; BMS: Research Funding; Incyte: Research Funding; Blueprint: Honoraria; Stemline: Honoraria; Taiho: Honoraria; Syntrix Pharmaceuticals: Research Funding. Sallman:Syntrix Pharmaceuticals: Research Funding; Incyte: Speakers Bureau; Takeda: Consultancy; Nemucore: Membership on an entity's Board of Directors or advisory committees; Intellia: Membership on an entity's Board of Directors or advisory committees; Lixte: Patents & Royalties: LB-100; Shattuck Labs: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Magenta: Consultancy; Aprea: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kite: Membership on an entity's Board of Directors or advisory committees; Syndax: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees. Lancet:Novartis: Consultancy; Syntrix Pharmaceuticals: Research Funding; Jasper Therapeutics: Consultancy; Dedham Group: Consultancy; Boxer Capital: Consultancy; Agios/Servio: Consultancy; Astellas: Consultancy; Jazz: Consultancy; Dava Oncology: Consultancy; BerGenBio: Consultancy; Millenium Pharma/Takeda: Consultancy; ElevateBio Management: Consultancy; Daiichi Sankyo: Consultancy; Celgene/BMS: Research Funding; AbbVie: Consultancy; Servier: Consultancy. Sweet:AROG: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Syntrix Pharmaceuticals: Research Funding; berGenBio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Curis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Mablytics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal